Rubberband IR Mixture 617
Rubberband IR Mixture 617
REF: 7227
£ 500
Model:
Quantity: 5000
Condition: New
Item Location:
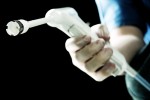
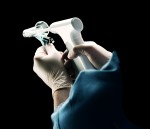
Ligature rings suitable for use in the medical device sector- they have been extensively tested for Cytotoxicity, Irritation and Sensitisation in accordance with International Standard ISO10993-1 and have passed all tests. They are moulded to take the form of a ‘ring’ shape and have an inner diameter of 1.8mm and an outer diameter of 4.8mm (bands will be supplied in this size). The bands have been supplied by a German company known as ‘Erchinger’, who are certified to ISO13485 and are known for their supply to the medical device sector. Currently, these bands are used on a device called the ‘Haemoband’ which is used for the treatment of haemorrhoids (a non-surgical treatment). The bands are loaded onto the ligator end of the Haemoband unit and, once the haemorrhoid has been targeted, the physician presses the trigger and a band fires, securing a band to the haemorrhoid. The next band is then automatically reloaded with the unit ready for the next band application. It is a non-sterile device and is disposable. Details of the product and procedure the bands are currently used with are given below. As the bands are currently not in a stretched state and can therefore have other uses. They are black in colour. Due to excessive ordering, our company now also have an excess of bands, which are currently being stored at the manufacturers.
If/once stretched for use medically, bands hold a 15 month shelf life. Expiry date stated as Dec 2016- gave as an example- was to be left blank but was a required field. N/A until bands stretched as this is when age testing was conducted.
Expiry date: December 4, 2016
Sellers Contact Details
- Seller Name: Lucie Morrison
- Seller Contact Number: 07891943079
- Seller Email Address: lmorrison@haemobandsurgical.com
Does the product comply with any of the following standard ISO/British Standard /DIN /EN/ RoHS/ FDA /Other?
Yes
Has the product been maintained within the manufactures guidelines in relation to Service/Maintenance/Calibration?
Yes
Are the manufacturers operating manuals, technical manuals, service manuals, directions for use available with this product?
Yes
Is the product still under manufacturers warranty?
No
Is a full range of spares available from the OEM (Original Equipment Manufacturer) relating to your product?
No
Is the OEM of this product still trading?
Yes
Is the product available on a PASA or any other NHS agreement?
No
Has this product been decontaminated (if applicable) within current guidelines?
N/A
Does the product conform to current WEEE regulations?
N/A